Ever since antiretroviral treatments against HIV infection were introduced in 1996, scientists have eagerly been hunting for a cure for the disease.
Although drugs can allow people who carry HIV to live normal, healthy lives, people who carry HIV have to take antiretroviral treatments for as long as they live. Little bits of virus can lie dormant in immune cells for years, so if patients stop taking the drugs, the dormant virus can come out of hiding and re-infect the patient.
Now, as the first people to start taking antiretroviral treatments are entering middle or old age, doctors are seeing side effects from inflammation caused by having even very low levels of circulating HIV. That’s yet another reason for developing a cure for the disease.
Now, researchers from the Norwegian University of Science and Technology’s (NTNU) Centre of Molecular Inflammation Research (CEMIR) have uncovered a previously unknown way in which the immune system can detect and respond to HIV infection, which could improve the chances of developing a cure. Their results have been published in Nature Communications.
Background
When HIV gets into the body, it infects the immune cells that the immune system uses to combat it—these are called cd4 T helper cells, or cd4 T cells.
Once the T cells are infected, they can’t protect the body from other diseases or infections. Hence, the illness caused by HIV infection is called acquired immune deficiency syndrome, or AIDS.
In a condition of full-blown AIDS, patients have almost no T cells, and die of any number of infections or diseases that do not afflict people with healthy immune systems. “Today we have treatments that can stop HIV from replicating itself, and the T helper cells can come back,” said Hany Zakaria Meås, a postdoc at CEMIR and a co-first author of the article. “You can live a perfectly healthy life, but you have to take medicine your whole life, because the day you stop treating it, the virus will come back.”
The virus recurs if antiretroviral therapy is stopped because HIV hides its genetic material inside T cells that are dormant. That means there’s always the potential for more virus to appear and wreak havoc. These reservoirs of virus have spurred a worldwide hunt for ways to shock or kick the virus out of the cells where it lies dormant.
If the dormant virus could be smoked out into the open, it could then be killed by the immune system or via drugs, leaving the patient HIV free, and cured, Meås said. “We need to activate the virus so it can start replicating, and that will make the cell visible to the immune system. That is the current idea for a cure. We just need to activate cells that are hiding away so we can kill them, while we give medicine that protects cells from infection, because there will be more virus produced.”
One such clinical trial in the U.K., called the RIVER study, tried this approach, but reported last year that the trial did not succeed, he said. HIV comes in different varieties, and not all varieties are equally able to infect all T helper cells. To infect a cell, the virus has to have to have a specific ligand that functions like a key and matches up with a specific receptor, like a lock, on the target cell.
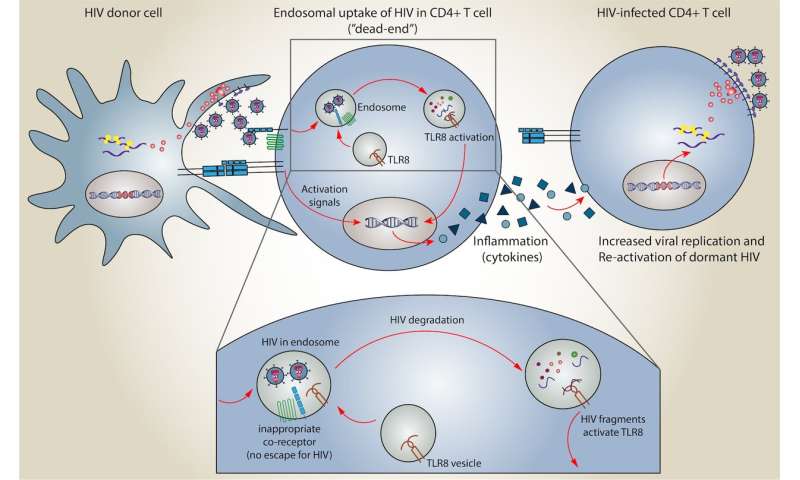
When a T helper cell doesn’t have the receptor that matches the ligand on the HIV virus, it can’t actively infect that T cell. Instead, the virus can get trapped in a vesicle in the cell called an endosome.

The CEMIR researchers studied what happened with uninfected T helper cells that had trapped HIV in endosomes. The uninfected cell responds to the HIV in the endosome by destroying the contents of the endosome, Meås said.
In the past, researchers have believed that this particular pathway—HIV being trapped in an endosome of a T helper cell, and the T helper cell destroying the contents of the endosome—was a kind of dead end for HIV infection. After all, the T helper cell hadn’t actually been infected, and the endosome destroyed the virus.
But now, the researchers have found a previously undescribed immune response that results from HIV destruction in the endosome. This response may hold the key to the shock-and-kill approach.
The researchers found that when the endosome destroys HIV, some of the genetic material is exposed to the T cell, which in turn activates a molecule called TLR8. This results in the production of substances called cytokines, which cause inflammation in the body.
One reason this is surprising is that T cells are part of the “adaptive” immune system, which learns and responds to specific infectious substances over time. There is also the lesser-known “innate” immune system that provides general immune protection by recognizing and responding to bits of viruses or bacteria that are common across many different viruses or bacteria.
TLR8 is a part of the body’s innate immune system. T cells are part of the adaptive immune system. Generally, it has been thought that these two systems were separate, independent branches. “In this study we show that a receptor associated with the innate system actually exists and functions in the adaptive immune system,” Meås said.
The inflammation caused by the cytokines wakes up T cells that had formerly been dormant and that contain HIV genetic material, said Markus Haug, a staff scientist at CEMIR and co-first author of the study.
“The T cell detects the virus and produces cytokines, and these cytokines act on cells that are properly infected with HIV and makes them produce more virus. The dormant T helper cells produce virus, and the T cells that were actively producing virus will produce more of it,” Haug said. In other words, the TLR8 signal and cytokine-induced inflammation shock the HIV out of the dormant cells so it can be destroyed.
The destruction of HIV by endosomes and the associated inflammation may also be one reason why patients who have been on antiretroviral treatments for decades are now beginning to develop inflammatory diseases more commonly associated with people decades older. These include dementia, cardiovascular diseases, metabolic syndrome and cancers that aren’t related to HIV.
Meås said that the mechanism whereby endosomes destroy HIV might release enough genetic material to trigger the T cell’s innate immune receptors to cause inflammation.
Beyond that, however, the findings do offer hope, said Jan Kristian Damås, a chief attending physician at the Department of Infectious Diseases, St. Olav’s Hospital, Trondheim University Hospital and an NTNU professor associated with CEMIR. Damås works with HIV patients and recruited the nine patients whose cells were used as part of the research. He is also an author on the paper.
“Today, we have very efficient drugs for suppressing HIV. However, we are not able to eradicate the virus, and just weeks after patients stop medication, the virus will reappear from virus reservoirs. Researchers and scientists believe that we can find a cure for HIV if we are able to eradicate these reservoirs,” Damås said.
Source: Read Full Article
