A novel gene editing technique developed by researchers at The Ohio State University Wexner Medical Center and College of Medicine is showing promise in correcting genetic mutations in Duchenne muscular dystrophy (DMD) and some cardiovascular diseases.
DMD is a deadly genetic disease caused by mutations to the dystrophin gene. Without dystrophin, muscle cells are damaged, resulting in progressive muscle weakness and wasting, which ultimately leads to respiratory impairment and heart failure. DMD is the most common form of muscular dystrophy and primarily affects boys. It occurs in about one out of every 5,000 male infants worldwide. The first symptoms usually start at age three and worsen over time, with many patients living only into their 20s.
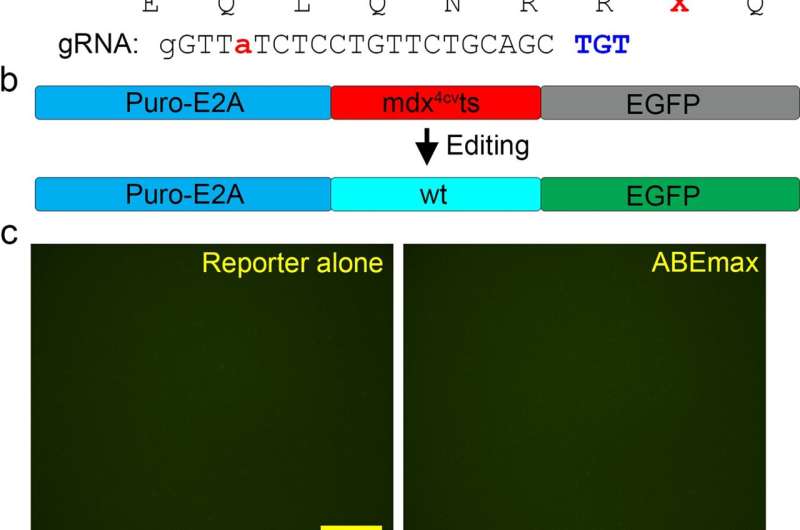
CRISPR genome editing has shown promise in correcting disease-causing mutations but can cut DNA, endangering human health. Ohio State researchers were successful in modifying a base editor—a technology built upon CRISPR—to safely and precisely edit a DMD mutation without cutting DNA in adult mice. Systemic delivery of an adeno-associated virus carrying the editor resulted in dystrophin restoration and functional improvement in the mice. The study’s results were published in Nature Communications.
“Our technique shows systemic in vivo base editing can be achieved with high efficiency in a preclinical relevant animal model of DMD,” said lead author Renzhi Han, a professor in the Division of Cardiac Surgery at the College of Medicine. “It has significant implications for clinical translation. This type of base editing does not require repeated treatment since it directly modifies the genetic code and produces a long-lasting therapeutic effect. Since it also doesn’t cut the genomic DNA, which happens with CRISPR, there are no large DNA deletions and chromosomal rearrangements.”
By modifying an adenine base editor to overcome various challenges, Han and his team were successful in restoring dystrophin expression in the muscle and hearts of mice with DMD. With a single intravenous injection of the improved adenine base editor at five weeks of age, over 95 percent of heart muscle cells at 10 months of age were restored to re-express dystrophin naturally. Researchers didn’t observe detectable adverse events in the treated animals.
“The editing efficiency in the heart was exceptionally high in the treated mice,” Han said. “Our base editing technique has tremendous potential because it could be used for targeting many other genetic diseases.”
Source: Read Full Article
