In a study, published in American Journal of Hematology, Mayo Clinic researchers examined telomere biology disorders, a group of rare genetic disorders characterized by short telomeres.
Telomeres are long segments at the end of chromosomes that protect the DNA from unraveling, similar to the small plastic sheath at the end of a shoelace. Telomeres shorten naturally with age but become abnormally shorter in telomere biology disorders. People with these disorders often have bone marrow failure and, for unknown reasons, a high risk of developing blood cancer.
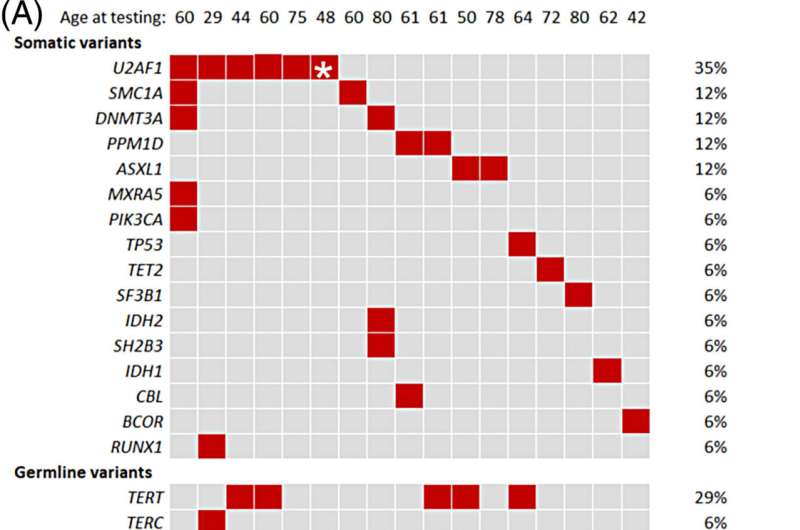
Mayo Clinic researchers explored the causes of this increased cancer risk by assessing clonal hematopoiesis in patients with telomere biology disorders. Clonal hematopoiesis is also an age-related event that occurs when blood stem cells acquire genetic mutations that make them divide more rapidly than normal. This creates a growing clone in the blood that can evolve into blood cancer. Approximately 10–20% of the population over 70 present an observable clonal hematopoiesis. However, how these clones evolve over time depends on the gene mutation acquired and the surrounding environment of the cells.
The study found that people with telomere biology disorders often have a high percentage of clonal hematopoiesis. Variants in the U2AF1 gene are especially common. In contrast, clonal hematopoiesis that occurs because of aging is associated with other mutated genes. This difference suggests that the U2AF1 gene may play a role in developing blood cancer, specifically in people with telomere biology disorders.
“U2AF1 mutations can affect the expression of specific genes and contribute to the emergence of clonal hematopoiesis in a way that is unique to these patients,” says Alejandro Ferrer, Ph.D., a Mayo Clinic hematology researcher. “We need to understand why this gene is so commonly mutated in people with this disorder and its role in cancer development.”
The findings are especially relevant for people with telomere biology disorders who may be sensitive to standard cancer therapies due to a combination of factors, including preexisting genomic instability, shortened telomeres and the potential effect of these therapies on cancer and healthy cells.
Researchers need to better understand why the U2AF1 gene is prominently mutated in people with telomere biology disorders compared to other genes. Assessing the clinical relevance may help advance knowledge and potentially develop targeted treatments tailored to their needs.
More information:
Alejandro Ferrer et al, Patients with telomere biology disorders show context specific somatic mosaic states with high frequency of U2AF1 variants, American Journal of Hematology (2023). DOI: 10.1002/ajh.27086
Journal information:
American Journal of Hematology
Source: Read Full Article
