The coronavirus disease 2019 (COVID-19) pandemic was triggered by the uncontrolled spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A year and a half later, the relationship between SARS-CoV-2 viral load and infectiousness remains a matter of scientific debate. Now, a new preprint research paper posted to the bioRxiv* server aims to quantify this relationship.
Viral load a crucial factor in spread
Earlier, respiratory droplets and aerosols containing the virus were considered to be the primary route by which the virus was spread. However, how does viral load modulate transmission by this route was another question which has not yet been formally established.
The problem begins with the fact that the virus mainly causes asymptomatic infection, and even with symptomatic cases, the majority of transmission occurs before the index patient is even diagnosed. At this point, therefore, viral load is entirely unrecorded.
However, viral load determines how infectious the patient is, influencing the time at which spread is most likely to occur, the chances of spread from a given case, and how interventions affect transmission. With the newly emerging variants of concern (VOCs), the relative impact of the viral load compared to other factors becomes still more important.
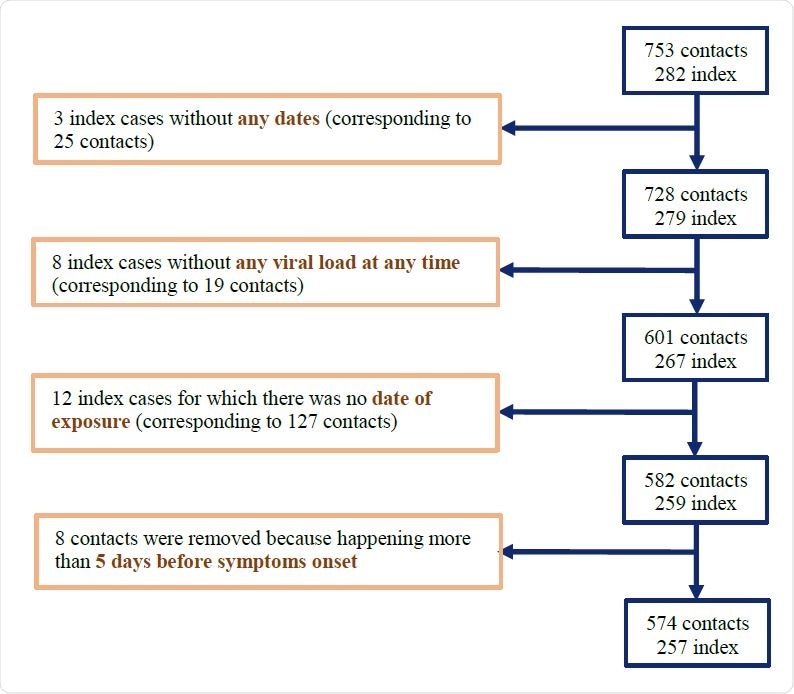
Earlier study provides data
A study on the effectiveness of hydroxychloroquine on SARS-CoV-2 transmission has examined the viral load at the time of spread in a cohort of 735 individuals at high risk of transmission from 282 index patients. All individuals were regularly monitored to understand the emergence of viral variants, the clinical course, and new infections among the contact cohort.
The higher the viral load of the infected person at the time of diagnosis, the greater was the chance that the high-risk contact with someone in the contact cohort would be followed by a new infection. This still does not show how the viral load at the time of contact is related to the risk of transmission – albeit it may be significantly more or less than that measured at the time of diagnosis.
This data was therefore re-analyzed using a different model so as to rebuild a graph of the viral load when contact occurred and to predict how this would affect transmission. The model was run with both a VOC and with immune-evading vaccines.
Study details
Almost three out of four index cases were female, with a median age of 42 years, with about 544 swabs being available. The swabs were taken at 0, 3, and 7 days from the start of the study, which occurred at a median of 4 days before the first swab was taken.
The highest viral load during follow-up was 8.4 log10 copies per mL.
Among contacts, just over half were female, median age 41 years, with contacts mainly being household contacts (60%). Over two-thirds of contacts occurred roughly one day after symptom onset. This was also the case with new infections, which followed 87 household and 29 non-household contacts, making up a quarter and a sixth of the total contacts, respectively.

Viral dynamics
The target cell limited model showed that the reproductive number R0, which quantified the number of cells infected by one infected cell, is 16, while less than one cell producing new viral particles is lost per day. Viral production is 4.1×105 per cell per day.
The peak viral load was predicted to be at the onset of symptoms, with the median value being 9.8 log10 copies per mL.
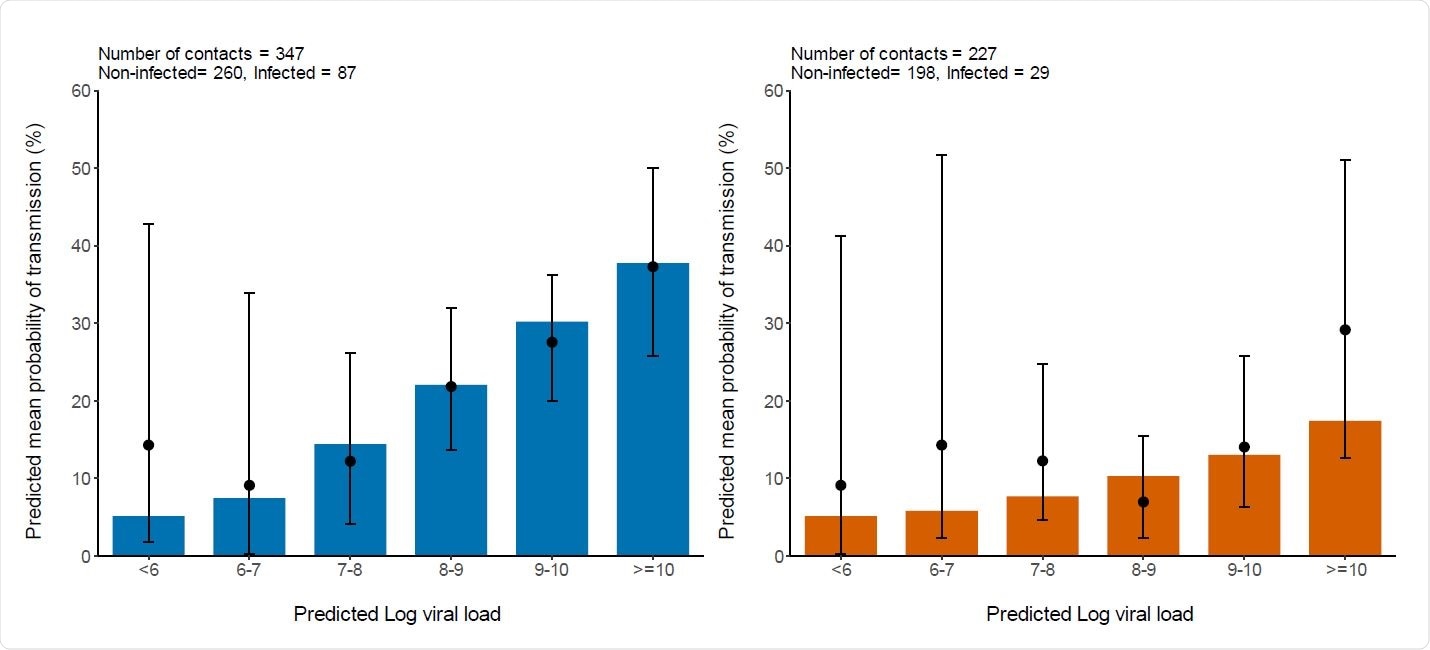
The model of probability transmission showed that viral spread was more likely after household contact with a case with a high viral load, but this was not so for non-household contacts. The average chances of transmission increased from the baseline value of 5%, correlated with the viral load when the viral load was less than 106 copies per mL.
With a viral load of 1010 copies per mL, up to 37% of household contacts, and 17% of non-household contacts, were likely to be infected. These estimates bear a striking similarity to the values of 37% and 29% obtained from the actual clinical study.
Again, despite significant differences between individuals, the transmission was most likely to occur at the onset of symptoms, with about 30% of household spread occurring at this time. With non-household contacts, the average value was 13%.
The scientists assumed that the chances of spread returned to baseline once the viral load fell below 6 log10 copies per mL. In these circumstances, the opportunity for transmission is much less than the period during which the patient continues to shed the virus, for a median infectious duration of 12 days.
Variants more infectious because of viral load
The model also used a contact profile similar to that in the original study to determine what changes occurred in viral transmission when the viral load changed. The researchers found that as with the study, the model predicted that a quarter and a tenth of household and non-household contacts, respectively, were likely to be infected.
As the rate of viral production is doubled, as seen following B1.1.7 strain infection, the risk of spread increases somewhat, to 27% and 12%, for household and non-household contacts. When doubled again, to fourfold the initial viral load, the figures become 29% and 13%.
At eight-fold the initial load, the chances of spread are 31% and 14%, respectively. When the Brazil P.1 and South African B1.1.351 variants are considered, the predicted estimates for viral transmission change from two- to ten-fold higher.
Vaccination reduces spread via viral load reduction
Since vaccination is supposed to reduce the viral loads, even if infection occurs, the researchers also examined the effects of lowering the number of viral particles. In fact, a 3.5-fold reduction has been reported in Israel.
At four-fold lower viral load, the average transmission rates would be 21% and 10% for household and non-household contacts, respectively. This is far greater than the 100-fold reduction observed in an actual study where all vaccinated individuals were tested for infection, whether symptomatic or not.
In that study, the likelihood of transmission was 12% and 7% – which is 50% and 38% less than the figures at baseline for household and non-household contacts, respectively.
What are the implications?
This is the first time that viral load has been examined in relation to infectiousness at this level of detail. The results show a significant effect in household contacts, with the chances of producing a second case being 37% if the index patient has a viral load over 10 log10 copies per mL.
The risk of secondary cases was linearly proportional to viral load, without evident saturation effects at high loads, unlike the predictions of theoretical models. However, individual differences were seen, with secondary infections occurring in 5% to 100% of contacts with different index patients – the median being 15%. This has been observed in earlier studies.
Since the UK B1.1.7 variant appears to have a cycle threshold (Ct) that increased by 1-2, on average, the researchers postulate that either this strain produces twice as many viral particles, or it is able to infect twice as many cells as the reference strain. The latter is suggested by the N501Y spike substitution, which leads to a higher binding affinity for the target cell receptor.
Overall, therefore, “we estimate that 2- to 4-fold increase in viral load level observed with B1.1.7 virus could lead to an increase in the probability of transmission by 8 to 17% after a high-risk contact.”
In either case, the viral load is increased by 2, which increases the chances of spread by 2%, or 8%, above baseline. The average likelihood of spread goes up to 17% above baseline when the viral load is increased four-fold.
This model assumes a relatively low effect of viral load; if it is actually much steeper, the new variants will cause a still more impressive rise in the number of secondary cases. Further studies will be required to validate these findings, taking the limitations of the current data into account.
However, vaccination appears to be highly protective as may be inferred from the five-fold to 100-fold lower viral loads recorded in infections following vaccination.
“This relationship can be used to predict the effects of changes in virus paradigm, caused by the emergence of new variants and/or the rollout of vaccination.”
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Marc, A. et al. (2021). Quantifying the relationship between SARS-CoV-2 viral load and infectiousness. medRxiv preprint. doi: https://doi.org/10.1101/2021.05.07.21256341. https://www.medrxiv.org/content/10.1101/2021.05.07.21256341v1
Posted in: Medical Research News | Disease/Infection News
Tags: binding affinity, Cell, Coronavirus, Coronavirus Disease COVID-19, CT, Hydroxychloroquine, Pandemic, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Virus

Written by
Dr. Liji Thomas
Dr. Liji Thomas is an OB-GYN, who graduated from the Government Medical College, University of Calicut, Kerala, in 2001. Liji practiced as a full-time consultant in obstetrics/gynecology in a private hospital for a few years following her graduation. She has counseled hundreds of patients facing issues from pregnancy-related problems and infertility, and has been in charge of over 2,000 deliveries, striving always to achieve a normal delivery rather than operative.
Source: Read Full Article
