Lipid nanoparticles (LNPs) are the carrier vehicles that protect messenger RNA (mRNA) molecules from degradation and aid intracellular delivery and endosomal escape in the nucleoside-modified mRNA-LNP vaccine platforms currently used by Pfizer/BioNTech and Moderna, against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). These two vaccines surpass the rest with their effective support for T follicular helper cells (Tfh) and the protective humoral responses observed in the preclinical trials.
While the vital role of LNPs in these vaccines' action is established, the potentially inflammatory nature of these LNPs is not assessed. Also, in the Pfizer/BioNTech and Moderna vaccines' human trials, it is reported that side effects often linked to inflammation, such as pain, swelling, fever, and sleepiness, are common.
Because the vaccine was presumed to be non-inflammatory, these side-effects were taken to be generated from the potent immune response to the vaccine. Therefore, there is a need for a systemic approach to analyze the inflammatory properties of LNPs and understand their role in the vaccination process.
In a recent bioRxiv* preprint research paper, Botond Z. Igyártó and colleagues from Thomas Jefferson University demonstrated the inflammatory nature of the lipid nanoparticles (LNPs). Upon intradermal administration in mice, they observed massive neutrophil infiltration, activation of diverse inflammatory pathways, and production of various inflammatory cytokines and chemokines. They observed similar observations when the LNPs were delivered intranasally.
Notably, intranasal inoculation with the same dose (10 μg) of LNP (as in the intradermal delivery) caused a high mortality rate in mice – about 80% of the treated mice died in less than 24 hours. The researchers found that the LNPs' inflammatory properties are not site-specific. They observed a fast diffusion, dispersion, and distribution rate in the tissues, upon internal delivery.
"Thus, the inflammatory milieu induced by the LNPs could be partially responsible for reported side effects of mRNA-LNP-based SARS-CoV-2 vaccines in humans, and are possibly contributory to their reported high potency for eliciting protective immunity."
The LNPs consist of a mixture of phospholipids, cholesterol, PEGylated lipids, and cationic or ionizable lipids. These improve structure, stability and support prolonged circulation. The cationic/ionizable lipids, complex with the negatively charged mRNA molecules and enable the exit of the mRNA from the endosome to the cytosol for translation.
It is reported that some LNPs containing ionizable/cationic lipids are highly inflammatory and possibly cytotoxic. A preclinical study showed adjuvant activity of mRNA complexed with LNPs. To avoid activation of innate inflammatory pathways, in the vaccine, the mRNA is nucleoside-modified.
In this study, the researchers noted that the inflammatory nature of the LNPs could explain their potent adjuvant activity and their superiority, compared to other adjuvants, in supporting the induction of adaptive immune responses.
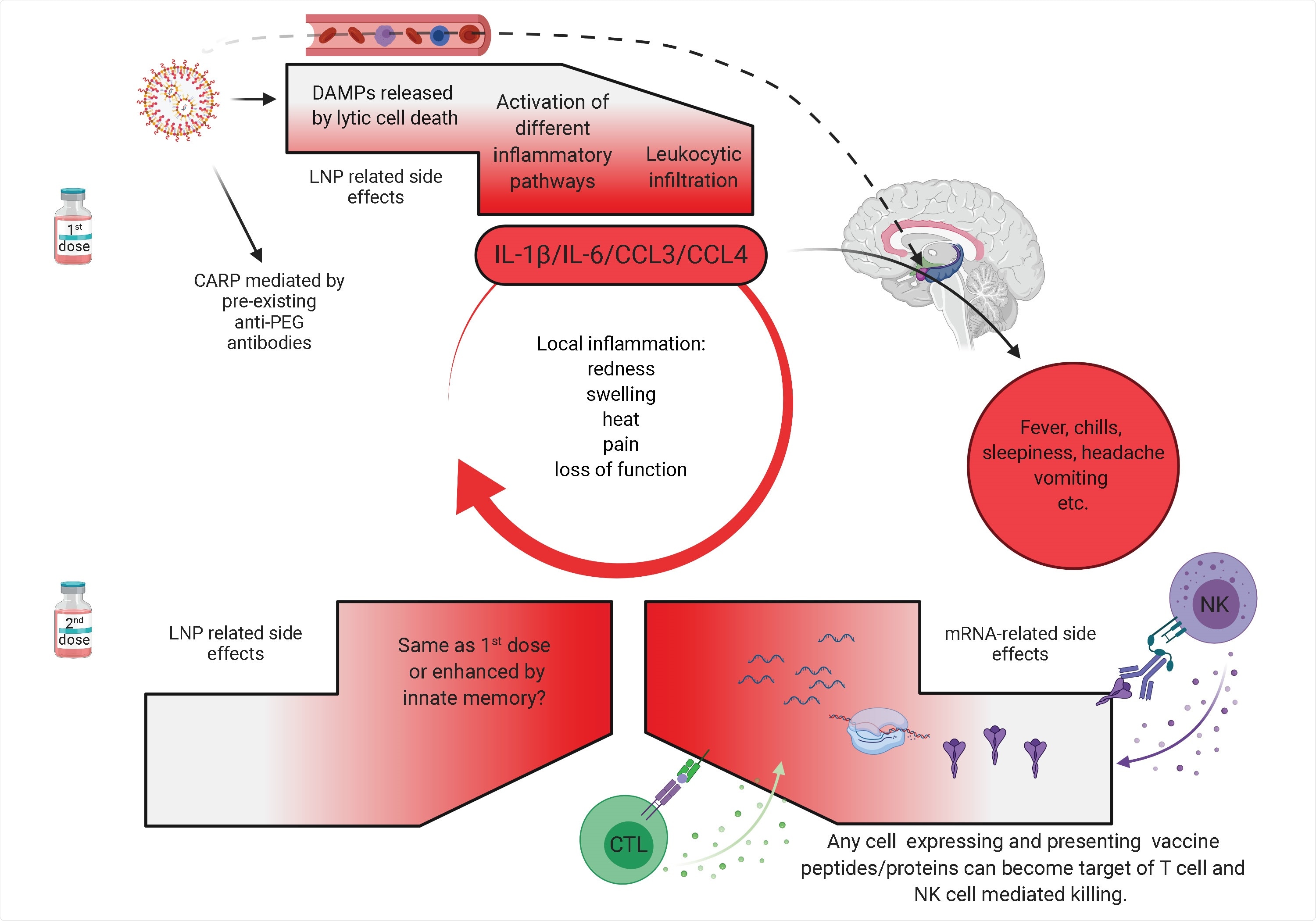
As expected with the observed side-effects in humans after intramuscular vaccination with the Pfizer/BioNTech or Moderna vaccines the intradermal inoculation of LNPs in mice led to secretion of the pyrogens – IL-1β/IL-6 and macrophage inflammatory protein-α (CCL3) and macrophage inflammatory protein-β (CCL4).
The researchers conducted Gene Set Enrichment Analysis (GSEA) to understand the pathways involved in the inflammatory cascade with the proprietary LNPs administered. The observed activated TLR pathways, among others, and upregulated inflammasome components such as Nlrp3 and enrichment of genes involved in necroptosis. In concordance, flow cytometry data revealed significant inflammatory cell death (after inoculation with LNPs), such as necroptosis and pyroptosis.
"Overall, the robust inflammatory milieu induced by LNPs, combined with the presentation of the vaccine-derived peptides/protein outside of antigen-presenting cells, might cause tissue damage and exacerbate side effects."
The researchers raised a concern that the LNPs, as lipid particles that can diffuse quickly, could potentially gain access to the central nervous system (CNS) through the olfactory bulb or blood. However, this needs to be determined with further study. Also, the role of innate memory responses to LNPs needs to be studied.
In conclusion, the intradermal inoculation with LNPs induced robust inflammation in mice. Using different techniques, the researchers showed that the LNPs, alone or complexed with control non-coding poly-cytosine mRNA, are highly inflammatory in mice – likely through the engagement and activation of various distinct and convergent inflammatory pathways.
Importantly, this study showed that the intradermal or intranasal delivery in mice of LNPs used in preclinical studies triggers inflammation characterized by leukocytic infiltration, activation of different inflammatory pathways and secretion of a diverse pool of inflammatory cytokines and chemokines.
However, further studies will be needed to determine the exact nature of the inflammatory responses triggered by mRNA-LNP vaccines in humans and how much overlap there might be with the inflammatory signatures documented here for mice, the researchers write.
"However, it will be necessary to strike a balance between positive adjuvant and negative inflammatory properties as LNP-associated vaccines move forward."
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory, Sonia Ndeupen, Zhen Qin, Sonya Jacobsen, Henri Estanbouli, Aurélie Bouteau, Botond Z. Igyártó, bioRxiv 2021.03.04.430128; doi: https://doi.org/10.1101/2021.03.04.430128, https://www.biorxiv.org/content/10.1101/2021.03.04.430128v1
Posted in: Medical Research News | Disease/Infection News
Tags: ADCC, Antibodies, Antigen, Blood, Cell, Cell Death, Central Nervous System, Chemokines, Cholesterol, Coronavirus, Coronavirus Disease COVID-19, Cytokines, Cytometry, Cytosine, Fever, Flow Cytometry, Gene, Genes, Hypothalamus, Immune Response, Inflammasome, Inflammation, Intracellular, Lipids, Macrophage, Medicine, Mortality, Nanoparticle, Nanoparticles, Necroptosis, Nervous System, Nucleoside, Pain, Peptides, Preclinical, Protein, Research, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Translation, Vaccine

Written by
Dr. Ramya Dwivedi
Ramya has a Ph.D. in Biotechnology from the National Chemical Laboratories (CSIR-NCL), in Pune. Her work consisted of functionalizing nanoparticles with different molecules of biological interest, studying the reaction system and establishing useful applications.
Source: Read Full Article
