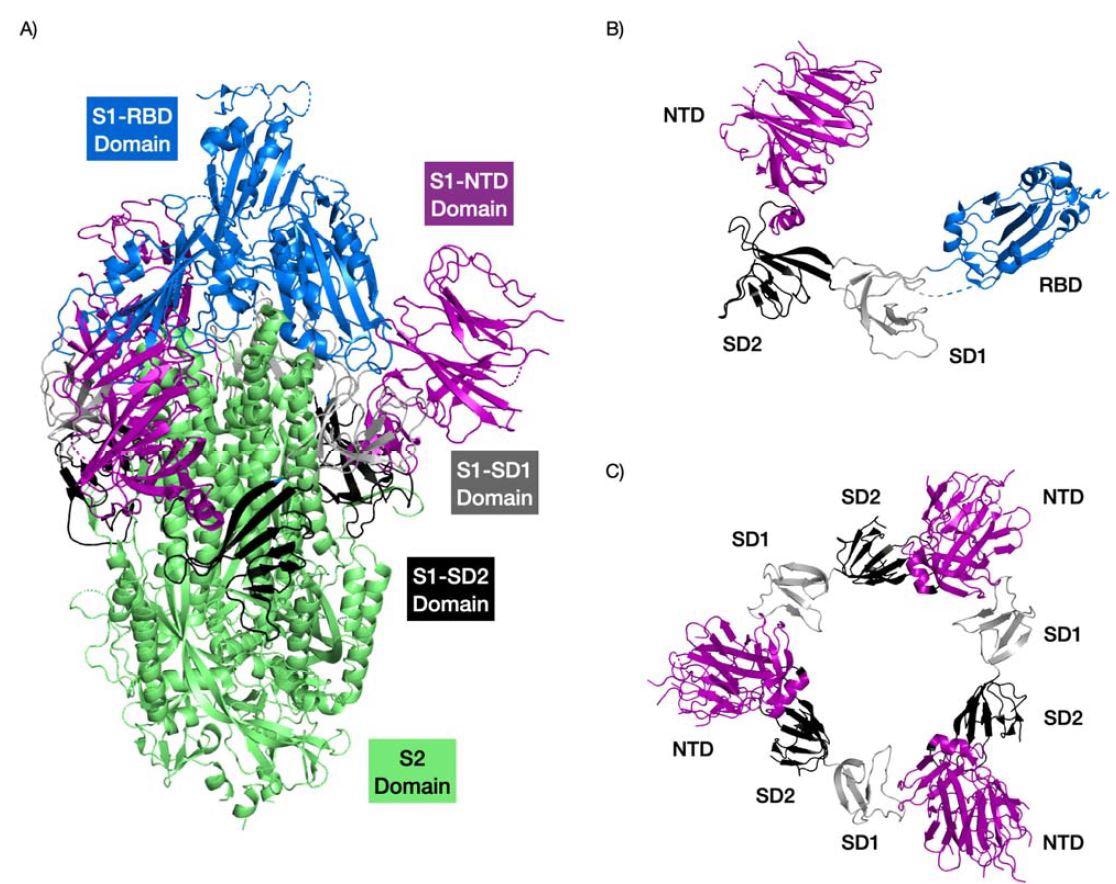
A majority of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) research focuses on the spike protein as it is crucial for viral entry and subsequent infection. Moreover, special attention has been given to the receptor-binding domain of the spike protein. However, another region called the N-terminal domain is also involved in viral entry into the host cell through sialic-acid receptor binding.
The N-terminal domain has a fold that can bind sugar, and new research led by Jonathan Lees from Oxford Brookes University found the sugar-binding pockets near the N-terminal domain help to increase viral infectivity. Furthermore, the findings show that the SARS-CoV-2 sugar-binding pockets are evolving with added loops observed in the pockets of new variants.
SARS-CoV-2 variants of concerns Gamma, Delta Plus, and Omicron have mutations in the N-terminal domain located near sugar-binding pockets and are associated with increased transmission. Understanding the structure of sugar-binding pockets on the N-terminal domain could help create new antiviral drugs.
The study “Insertions in the SARS-CoV-2 Spike N-Terminal Domain May Aid COVID-19 Transmission” was recently posted to the bioRxiv* preprint server.
 Study: Insertions in the SARS-CoV-2 Spike N-Terminal Domain May Aid COVID-19 Transmission. Image Credit: NIAID
Study: Insertions in the SARS-CoV-2 Spike N-Terminal Domain May Aid COVID-19 Transmission. Image Credit: NIAID
Pockets in the N-terminal domain have strong binding to sugar
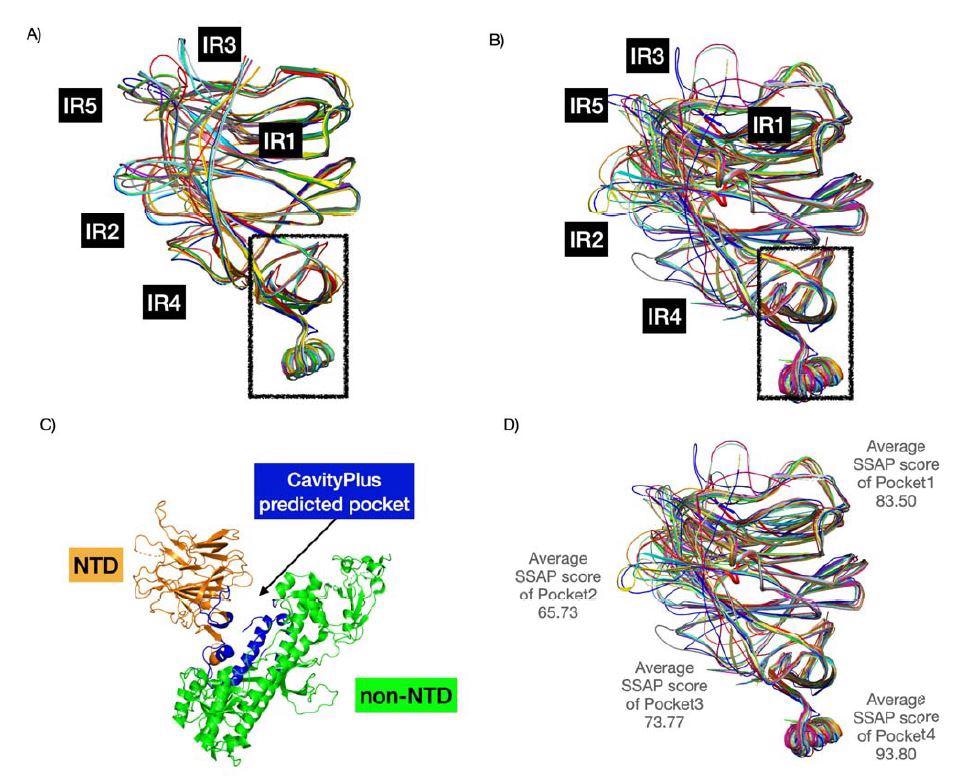
The researchers analyzed four binding pockets in the SARS-CoV-2 N-terminal domain along with the N-terminal domain of other coronaviruses.
Findings showed that the second and third pockets had greater binding towards sialic acid than the first sugar-binding pocket.
The binding strength of the second pocket differed across all studied coronaviruses. However, the third pocket retained strong binding to sialic acid.
Additionally, insertions in the N-terminal domain contributed to more loops that extended the first pocket. The end result was increased contact and binding with sialic acid. Other regions in the N-terminal domain near the sugar-binding pockets also enhanced sugar-binding interactions.
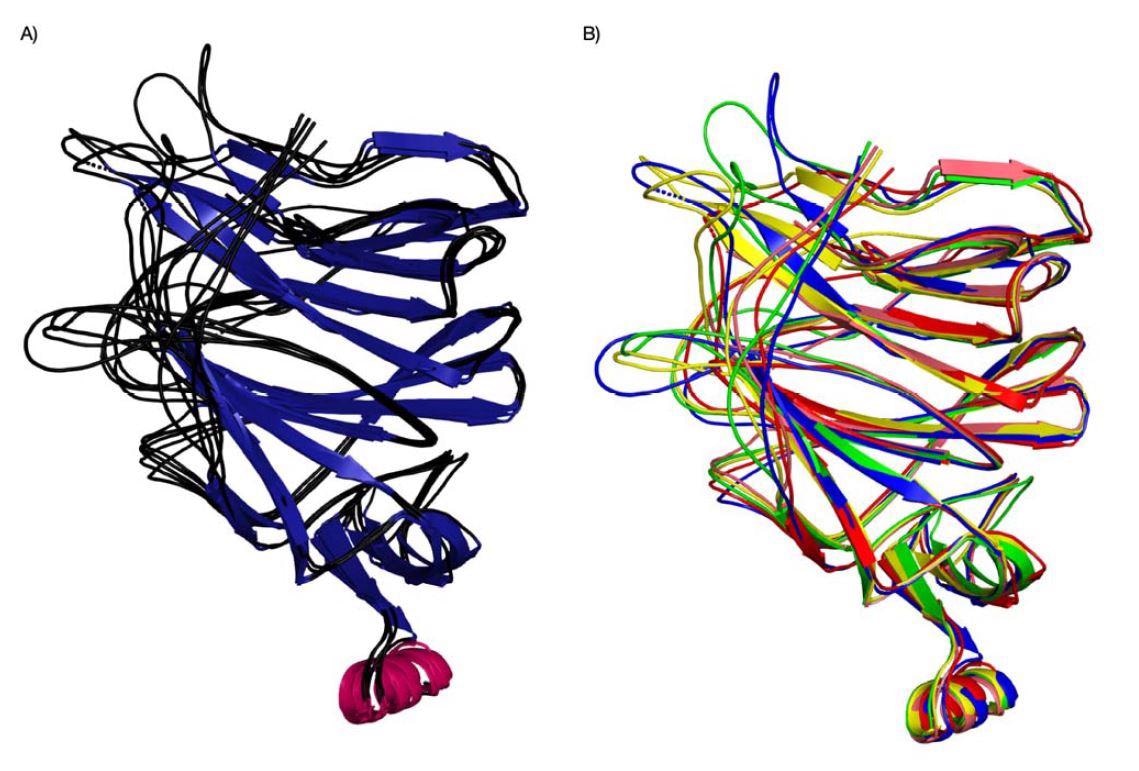
Evolution of binding pockets in newer coronavirus strains
Pockets 2 and 3 were more diverse in their structure. Additionally, the emergence of the IR2 indel region — an area with a sugar-binding motif for sialic acids — in the N-terminal domain is able to interact with pockets 2 and 3 because of the added loop. The findings suggest the SARS-CoV-2 virus is evolving in these areas to improve sialic acid-binding and increase infectivity.
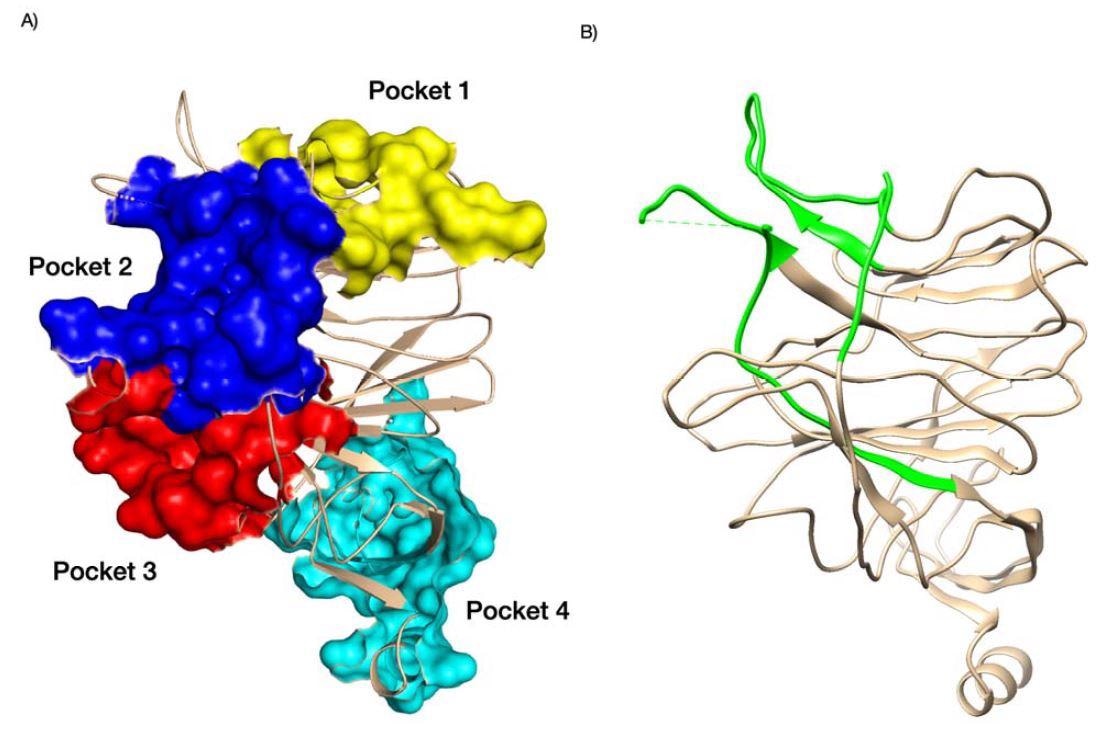
The researchers hypothesize that the SARS-CoV-2 virus may be evolving specifically in these areas as a response to specific sugar modifications in human cells. Indeed, binding energy studies showed that some SARS-CoV-2 variants of concern had increased binding to sugars in pocket 3.
Binding affinity to sugar differ across coronaviruses and SARS-CoV-2 variants of concern
The researchers conducted a computational analysis to measure the binding energy of coronaviruses to sialic acid.
Their results found stronger binding activity in the SARS-CoV-2 N-terminal domain compared to SARS-CoV. Specifically, binding was strongest in binding pockets 1 to 3.
Compared to SARS-CoV and the original SARS-CoV-2 strain identified in Wuhan, China, the Kappa variant with the E154K mutation had stronger binding in pocket 1. In addition, the Delta, Iota, and Mu variants also had stronger binding in pocket 3 when they had the T95I mutation.
T95I mutations are one of the newer mutations found in the Delta plus variant.
An interesting observation to the researchers is that the distance between mutations to the sugar-binding pockets can influence binding to other regions near the pocket even if they are not directly binding to sialic acid.
The Gamma, Omicron, and Delta Plus variants have high binding energy in the N-terminal domain binding pockets 1 and 3. This may be linked to the increased level of transmission observed with these variants of concern.
“We propose continuous monitoring of NTD mutations and indels in the context of newly emerging variants and their impacts on sugar-binding,” wrote the research team. “For example, the Omicron variant has a rather unique 3 amino acid insertion at position 214 which lies near to pocket 3.”
The high conservation and druggable nature of pocket 1 make it a suitable target for the development of drugs that would reduce or block binding to sialic acid. The researchers note that targeting galectins and the sugar-binding pockets could help prevent infectivity into the host cell and influence the immune response towards the virus.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Lam SD, et al. (2021). Insertions in the SARS-CoV-2 Spike N-Terminal Domain May Aid COVID-19 Transmission. bioRxiv. Doi: https://doi.org/10.1101/2021.12.06.471394, https://www.biorxiv.org/content/10.1101/2021.12.06.471394v1
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: Amino Acid, binding affinity, Cell, Coronavirus, Coronavirus Disease COVID-19, Drugs, Evolution, Galectins, Immune Response, Mutation, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Virus

Written by
Jocelyn Solis-Moreira
Jocelyn Solis-Moreira graduated with a Bachelor's in Integrative Neuroscience, where she then pursued graduate research looking at the long-term effects of adolescent binge drinking on the brain's neurochemistry in adulthood.
Source: Read Full Article
